Introduction: Fibrin degradation products, D-Dimer and X-oligomers, are biomarkers for activation of coagulation. D-Dimer testing is recommended as an additional diagnostic tool for proximal deep vein thrombosis (DVT) and pulmonary embolism (PE), in conjunction with clinical presentation and pre-test probability (Lim, et al. Blood Adv. 2018). D-Dimer levels naturally increase in patients over the age of 50 years, which reduces the specificity of D-Dimer levels as a diagnostic aid for proximal DVT and PE in older patients. We conducted a study to assess the diagnostic accuracy of the Tina-quant® D-Dimer Gen.2 (D-DI2; Roche Diagnostics) assay in patients with a low or intermediate pretest probability for proximal DVT or PE (Bertsch, et al. ISTH 2020; abstract PB0600). An exploratory objective of the study was to evaluate the diagnostic accuracy of the D-DI2 assay in the total study population after applying an age-adjusted cut-off value for patients aged >50 years.
Methods: This prospective, observational, multi-center study was conducted between July 2017 and August 2019. Samples were collected at six European hospitals or specialist referral centers, or purchased from a commercial vendor (BioPartners Inc.). The 3.2% citrated plasma samples were analyzed at a central site using the D-DI2 assay on the cobas t 711 analyzer (Roche Diagnostics). Eligible patients were aged ≥18 years, presenting with symptoms suggestive of proximal DVT and/or PE, and with a low or intermediate pretest probability of DVT or PE by Wells score. Main exclusion criteria included DVT and/or PE symptoms for >7 days or a previous diagnosis of DVT and/or PE. DVT and/or PE were diagnosed according to local imaging protocols and standard-of-care procedures, in line with current clinical guidelines. Patients were followed-up for ≥90 days after hospital discharge to verify the clinical diagnosis and record any adverse events. In the primary analysis, a D-DI2 assay cut-off value of 0.5 μg fibrinogen equivalent units (FEU)/mL was applied across all patients, regardless of age. For the purpose of this exploratory analysis, age-adjusted cut-off values were calculated by multiplying age by 0.01 μg FEU/mL for patients aged >50 years.
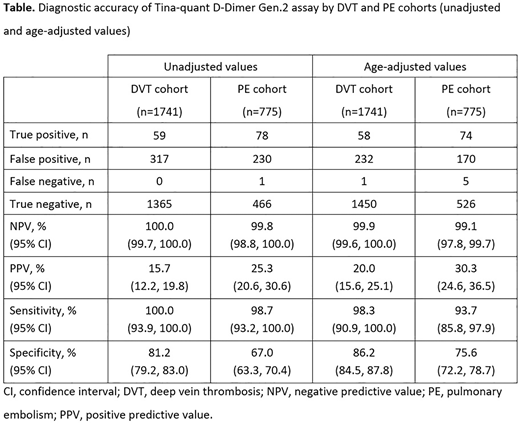
Results: In total, 2516 patients were included in the analysis (1741 DVT cohort; 775 PE cohort); of these, 1538 patients were aged >50 years (996 DVT cohort; 542 PE cohort) and the D-DI2 assay cut-off values for these patients were age-adjusted accordingly. The parameters for diagnostic accuracy for both the DVT and PE cohorts, unadjusted and age-adjusted values, are provided in the Table. Both negative predictive value (NPV) and sensitivity remained high after adjustment for age across both cohorts; however, there were slight decreases compared with the unadjusted values. Positive predictive value (PPV) was increased in both cohorts after age adjustment when compared with the unadjusted values. A reduction in false positives were reported for both the DVT (27%) and PE (26%) cohorts when adjusted for age compared with the unadjusted values. After age-adjustment, the number of false negatives in the total study population increased slightly in the DVT cohort (from zero to one case) and in the PE cohort (from one to five cases). Overall, specificity was increased when the results were adjusted for age.
Conclusions: In our study population, when used in conjunction with a low or intermediate pretest probability for DVT and/or PE according to Wells criteria, the D-DI2 assay identifies patients at very low risk for proximal DVT and PE. Adjustment of the cut-off value for age resulted in a slight decrease in NPV, though still below the diagnostic threshold. The benefit of a reduction in false positives observed after age adjustment has the potential to prevent further unnecessary diagnostic procedures for patients.
Bertsch:Nuremberg General Hospital/Paracelsus Medical University: Current Employment. Blaschke:BMBF: Research Funding; Innovationsfonds: Research Funding; DGINA: Membership on an entity's Board of Directors or advisory committees. Body:University of Manchester: Current Employment; Beckman Coulter: Consultancy; LumiraDx: Consultancy; Roche Diagnostics: Consultancy; Siemens Healthineers: Consultancy; Abbott Point of Care: Consultancy; Abbott Point of Care: Research Funding; Roche Diagnostics: Research Funding; American Association of Clinical Chemistry (sponsored session from Roche, Abbott, ET Healthcare, Ortho, Siemens, Beckman): Speakers Bureau; LumiraDx advisory committee: Membership on an entity's Board of Directors or advisory committees; Creavo (chair of Trial Steering Committee): Membership on an entity's Board of Directors or advisory committees. Davidson:Roche Diagnostics International: Current Employment. Rieger:Roche Diagnostics GmbH: Current Employment. Horner:Salford Royal NHS Foundation Trust: Current Employment. Sonner:Roche Diagnostics GmbH: Consultancy; TRIGA-S Scientific Solutions: Current Employment. Sun:Roche Diagnostics GmbH: Current Employment. Turnes:ICON Clinical Research UK Ltd: Current Employment. Hoffman:Roche Diagnostics GmbH, Germany: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.